Ghana To Commence Implementation Of New Malaria Vaccine
- Home
- Ghana To Commence Implementation Of New Malaria Vaccine
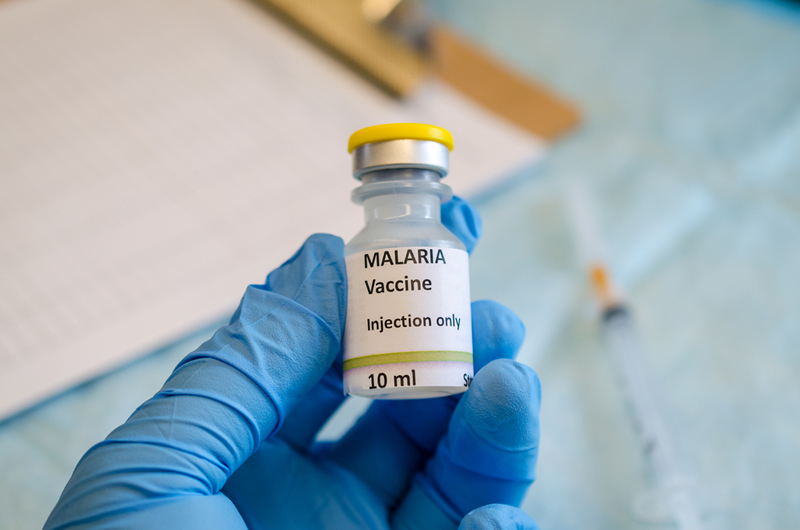
Ghana To Commence Implementation Of New Malaria Vaccine
 Ghana would from May 1, 2019, commence the pilot implementation programme and post market studies of the new malaria vaccine by name RTS, S/AS01, also known as Mosquirix.
Ghana would from May 1, 2019, commence the pilot implementation programme and post market studies of the new malaria vaccine by name RTS, S/AS01, also known as Mosquirix.
The RTS, S/AS01 Malaria Vaccine Development Programme (MVIP), which has travelled a 30-year journey, starting from 1998, would finally bring hope to millions of children globally, by reducing the frequency of infection, and their chances of getting severe malaria.
Dr Anthony Nsiah-Asare, the Director-General of the Ghana Health Service (GHS), who announced this at a press briefing in Accra on Thursday, said the pilot implementation of the Vaccine would be launched in Cape Coast in the Central Region on April 30, 2019.
He said the vaccine would be given in four doses from the age of six months, seven, nine and 24 months respectively, as an injection on the left thigh by a trained health worker, and that a child must receive all the four doses to get the most protection.
Hence all eligible children will be targeted to receive their full doses of RTS, S/AS01 delivered through existing routine strategies alongside other vaccines in the Expanded Immunisation schedules.
The Director-General explained that the MVIP would be done alongside with Kenya and Malawi, with the goal to assess the feasibility and impact of introducing the RTS, S/AS01 into the routine immunization programme while monitoring safety.
It was also expected to be an additional tool of intervention to accelerate countries efforts towards control and elimination of the disease.
The press briefing was to inform the media and Ghanaians of the preparations and progress made so far towards the introduction of the MVIP and the next steps.
Dr Nsiah-Asare assured Ghanaians of the safety of the new vaccine saying, from the various trials, the RTS, S/AS01 has been observed to have a very good safety profile, good efficacy and impacted positively on the health of children, following which the World Health Organisation (WHO) recommended the pilot implementation of the vaccine within the routine immunization programme of the country.
He said the Food and Drugs Authority (FDA) had authorised for the vaccine to be used in the targeted areas, saying six regions made up of the Volta, Oti, Ahafo, Bono, Bono East and Central, would participate in the pilot implementation programme, within which 33 districts would receive 400,000 doses of the malaria Vaccine as the first batch, with a gradual scale up later to other districts.
He said Ghana’s target was to reach between 120,000 to 150,000 children per year for the next two to three years, and to avert at least 50,000 malaria cases and save approximately 750 children from dying from malaria in intervention areas.
The Ministry of Health and its Agencies together with the partners have initiated a number of preparatory activities such as staff training, the development of a Communication’s Strategy including (crisis communication strategy), and materials such as flyers, posters, flip charts and leaflets which have been pretested and approved by the relevant committees of the GHS and FDA to ensure the safe introduction of the MVIP, he said.
He said the GHS has also started a thorough stakeholders’ engagement with the Cabinet, Parliament, Ghana Academy of Arts and Sciences, Professional Health Groupings, some Media Editors, the Health Management Leadership of the targeted regions and Health Professional Associations, assessed and strengthened the capacity of the country’s cold chain and injection waste management, as well as the procurement of the Vaccines by taking delivery of the first batch of 400,000 doses.
He said as with new vaccines, and in line with national regulations, the GHS would continue to monitor the safety profile of the RTS, S/AS01 as done for all other vaccines within the routine immunization programme, and thanked all the partners especially the WHO for their assistance.
Dr Badu Sarkodie, the Director of the Public Health Division of the GHS, noted that with the malaria burden remaining high despite the significant gains made in the various intervention strategy over the years, the RTS, S/AS01 would stimulate the body’s own immune system to defend against the parasite and prevent it from infecting, maturing, and multiplying in the liver.
He explained that the Phase three trial was conducted from 2009 to 2013, with a total of 15,499 children in 11 sites and seven African Countries, with Ghana using its Kintampo and Agogo Sites.
Source: GNA
- Share
Classic Ghana
Classic Ghana brings you into a fun world of arts, entertainment, fashion, beauty, photography, culture and all things in between. Let’s explore these together!